This is a plain English summary of an original research article. The views expressed are those of the author(s) and reviewer(s) at the time of publication.
Researchers developed a tool that allows people to rate their concern about common side effects of antidepressant and antipsychotic medicines. The tool ranks potential treatment options in a visual display that highlights the drugs with side effects of least concern to individual patients.
This work could inform shared decision-making between clinicians and people considering these treatments.
More information about antidepressant and antipsychotic medications can be found on the NHS website.
The issue: which is the right antidepressant or antipsychotic for me?
In the 5 years to June 2023, around 6.7 million people in England were prescribed antidepressants, and around 650,000, antipsychotic drugs.
Most (75%) people taking these medications experience some side-effects (drowsiness, nausea or dry mouth, for instance). As a result, some do not take their medications as prescribed, which increases their risk of relapse. Most (73%) people with first-episode psychosis, and some (1 in 10) with a new episode of depression, change or discontinue their medication.
Clinical guidelines from the National Institute for Health and Care Excellence (NICE) recommend shared decision-making in which clinicians and patients discuss the side effects of antidepressants and antipsychotics before prescribing. But time may be too limited in consultations to consider all possibilities.
Different drugs have different side effects. Individual people may be more concerned about the likely side-effects of one drug (weight gain, for instance) than of another (tremor, for instance). Clinicians aim to help each patient find the most acceptable treatment.
Researchers explored whether digital tools could aid these conversations by simplifying information on side effects.
What’s new?
Researchers brought together information on depression, schizophrenia, antidepressants and antipsychotics from guidelines (national and international) and studies. They collated information on recommended treatments for depression and schizophrenia, and the recognised side effects.
The database they created included 32 antipsychotics (with 14 side effects) and 37 antidepressants (with 9 side effects).
To help clinicians navigate the databases and personalise treatment choices, the researchers developed a tool called the Psymatik Treatment Optimizer. People rate their concern about each side effect by moving a dial on a sliding scale, from minor to major concern. The tool displays their results as a heatmap (see below). Red shows most concern about a side effect, yellow shows least concern; each row corresponds to a different drug. In the example below, the heatmap suggests that drug 3, at the top, is least likely to give the user the side effects they are most concerned about.
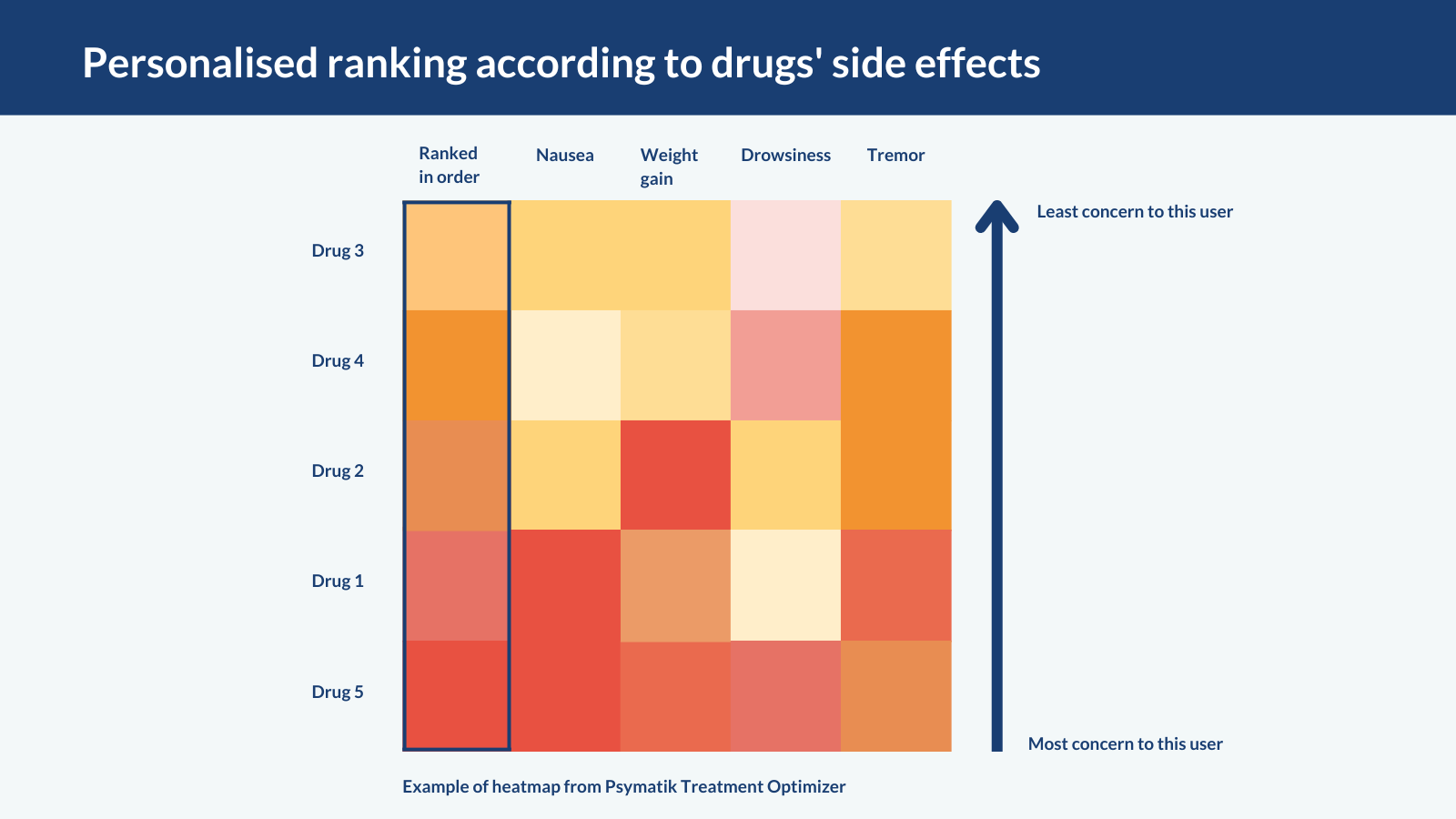
The authors also ranked drugs according to how effectively they treat depression or psychosis. This ranking appears as a separate column on the heatmap, to further help shared decision-making in consultations.
Why is this important?
People with lived experience of mental health conditions told researchers that traditional consultations could leave them feeling unempowered and uninformed. This tool, which gives clinicians quick sight of drugs’ side effect profiles, could inform shared decision-making during short consultations. An evidence-based choice of drug could reduce the burden of side effects people are most concerned about, and encourage them to take their medication as prescribed.
The current version of the tool has limitations; it does not include information on patients’ age, ethnicity or sex, which might make individual people more susceptible to some side effects. It also does not account for medication dose, other medications or lifestyle factors (smoking, for instance). In addition, side effects were not main outcomes of the trials that fed into the databases used.
Even so, given the high rates of side effects reported and the large number of trial participants, the researchers are confident in the data quality.
What’s next?
The tool (called Psymatik) will be evaluated further to determine its in real-world settings to determine its impact on patient satisfaction, quality of life and service use, and whether it is good value for money. The side effect databases will be updated as new side effect information becomes available.
You may be interested to read
This is a summary of: Pillinger T, and others. Antidepressant and antipsychotic side-effects and personalised prescribing: a systematic review and digital tool development. Lancet Psychiatry 2023; 10: 860 – 876.
An Editorial about the Psymatik tool: Asmal L, Kredo T. Balancing complexity and accessibility with the Psymatik Treatment Optimizer. Lancet Psychiatry 2023; 10: 821 – 823.
Funding: This study was funded by the NIHR.
Conflicts of Interest: Several of the study authors have received funding from pharmaceutical companies. See paper for full details.
Disclaimer: Summaries on NIHR Evidence are not a substitute for professional medical advice. They provide information about research which is funded or supported by the NIHR. Please note that the views expressed are those of the author(s) and reviewer(s) at the time of publication. They do not necessarily reflect the views of the NHS, the NIHR or the Department of Health and Social Care.
NIHR Evidence is covered by the creative commons, CC-BY licence. Written content and infographics may be freely reproduced provided that suitable acknowledgement is made. Note, this licence excludes comments and images made by third parties, audiovisual content, and linked content on other websites.